General
As a rule, foods can be placed on the European market without prior approval. Novel foods and food ingredients are an exception, as there is no sufficient experience base with regard to their safety and tolerance. This includes both newly developed and innovative foods, as well as foods produced using new technologies and production processes, and foods traditionally consumed outside the EU but not in Europe.
Definition of novel foods
Novel foods are all foods that have not been used for human consumption to a significant degree within the European Union before 15 May 1997, irrespective of the dates of accession of Member States to the Union, and fall into at least one of the following 10 food categories:
- with a new or intentionally modified molecular structure (e.g. tagatose, salatrim)
- consist of or are isolated from microorganisms, fungi or algae (e.g. algae oil from the microalgae Ulkenia sp. )
- consist of or are isolated from materials of mineral origin (e.g. clinoptilolite (zeolite))
- consist of or are isolated from plants and parts of plants (e.g. noni juice (Morinda citrifolia), chia seeds (Salvia hispanica))
- consist of or have been isolated from animals or their parts (e.g. insects, oil from Antarctic krill (Euphasia superba), peptides from the fish Sardinops sagax)
- cell and tissue cultures from animals, plants, microorganisms, fungi or algae (e.g. extract from cell cultures of Echinacea angustifolia, in vitro meat)
- food resulting from a production process not used for food production within the Union before 15 May 1997 resulting in a change in composition or structure (e.g. high pressure pasteurised fruit preparations, UV-treated mushrooms (Agaricus bisporus), UV-treated baker's yeast (Saccharomyces cerevisiae), UV-treated milk)
- consist of engineered nanomaterials (according to Article 3, Para. 2, lit f)
- vitamins, minerals and other substances (e.g. iron (II) ammonium phosphate, vitamin K2 (menaquinone), chromium picolinate)
- used exclusively in food supplements (not permitted in food categories other than food supplements) (e.g. maqui berry (Aristotelia chilensis), rose root (Rhodiola rosea)
Legal basis
Novel foods are regulated in the Novel Food Regulation (EU) 2015/2283. The exact definitions of the food categories are listed in Article 3(2) of the regulation.
Food additives, food flavourings, food enzymes, genetically modified food and extraction solvents for the production of food are not novel foods, as they are subject to their own legal regulations (according to Article 2, Paragraph 2 of Novel Food Regulation (EU) 2015/2283).
Novel foods are safe
Novel foods must be subject to a uniform safety assessment before they can be placed on the market in the EU. Novel foods must not pose a risk to the consumer and must not be misleading. Furthermore, they must not differ from the conventional foods and food ingredients they are intended to replace in such a way that their normal consumption would result in nutritional deficiencies for the consumer.
Clarification of the Novel Food Status
The food business operator is responsible for verifying whether the food to be placed on the market is a novel food. To clarify the Novel Food status, it is recommended to consult the Union list (Implementing Regulation (EU) 2017/2470 as amended consolidated version) as well as the Novel Food Catalogue of the European Commission. The Novel Food Catalogue of the European Commission provides information on the Novel Food status of foods and ingredients. Since 01 January 2018 there is the Union List, a positive list in which all approved Novel Foods are listed. If a Novel Food is already listed in the Union List, it can be placed on the market under compliance with the conditions of use and specifications. Another aid for clarifying the Novel Food status are the German Substance Lists („Deutschen Stofflisten“), which are intended to provide an overview of the use of plants, fungi and algae in foodstuffs.
For determining the criterion "significant consumption before 15 May 1997", the guideline "human consumption to a significant degree" published by the European Commission is used.
In case of existing uncertainty as to whether the food is an unauthorized novel food, the food business operator may consult the competent authority of the Member State in which the potentially novel food is to be placed on the market first (= Consultation procedure according to Article 4 of Novel Food Regulation (EU) 2015/2283).
In Austria, the Federal Ministry of Social Affairs, Health, Care and Consumer Protection (BMSGPK) is the competent authority. For the determination of the status, relevant information has to be submitted. For this purpose, procedural steps for consultation as well as further requirements for the information to be provided have been laid down (Implementing Regulation (EU) 2018/456).
Contact and information on questions for the Austrian market: Federal Ministry of Social Affairs, Health, Care and Consumer Protection (BMSGPK) Department III/A/6, novelfood@gesundheitsministerium.gv.at
The results of the Europe-wide consultation processes (Implementing Regulation (EU) 2018/456 on the procedural steps for consultation on the determination of novel food status) are publicly available online at the European Commission.
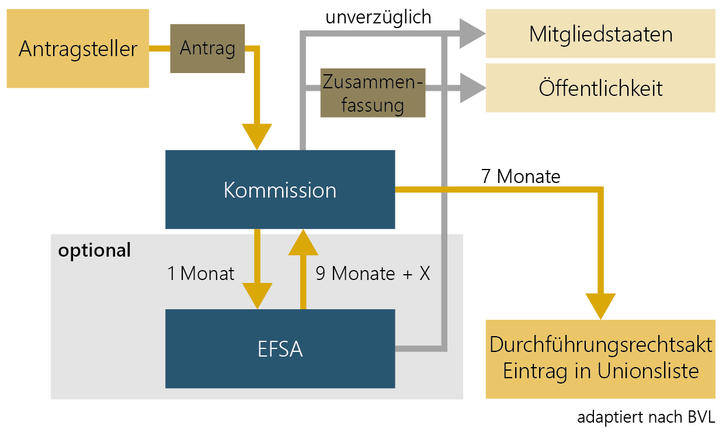
Authorisation of a Novel Food
If the novel food is not included in the Union list, an authorisation must be submitted to the European Commission in accordance with Article 10 of Novel Food Regulation (EU) 2015/2283.
In this new procedure, the member states are only informed, the evaluation and authorisation is the responsibility of the Commission and EFSA. EFSA has published an application guidance document and checklist for the applicant.
In doing so, the manufacturer shall submit to the Commission, via the E-Submission Food Chain Platform, the necessary documents as defined in the Implementing Regulation (EU) 2017/2469 laying down administrative and scientific requirements for the applications pursuant to Article 10.
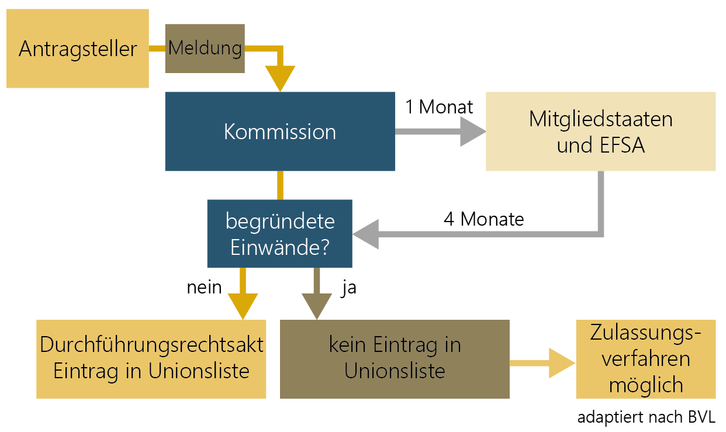
Notification of a traditional food from third countries
There is facilitated market access into the EU for traditional foods from third countries. However a safe history of use of at least 25 years outside the EU has to be proven. But this only applies to plants, animals, micro-organisms, fungi, algae and cell and tissue cultures.
If there are no objections to the notification of the traditional food, it is entered on the Union list by means of an implementing act. In case of safety concerns, an authorisation procedure with shorter deadlines is possible (Article 16). EFSA has also published guidance on the notification of traditional foods from third countries. The procedure for notification of a traditional food is regulated in the Implementing Regulation (EU) 2017/2468.
Currently ongoing applications for authorisation of a novel food as well as a traditional food from third countries can be viewed online at the European Commission.
More information about Novel Foods
Information of the European Commission on Novel Foods
Union list - enumeration of all approved novel foods (It is recommended to use the current consolidated version.)
Information from the European Food Safety Authority on Novel Foods
EFSA Publication: Ververis, Ermolaos & Reinhard, Ackerl & Azzollini, Domenico & Colombo, Paolo & Agnès, de & Céline, Dumas & Antonio, Fernandez-Dumont & Lucien, Ferreira & Germini, Andrea & Tilemachos, Goumperis & Eirini, Kouloura & Leonard, Matijevic & Precup, Gabriela & Roldan Torres, Ruth & Annamaria, Rossi & Roman, Svejstil & Emanuela, Turla & Wolfgang, Gelbmann. (2020). Novel Foods in the European Union: scientific requirements and challenges of the risk assessment process by the European Food Safety Authority. Food Research International. 109515. 10.1016/j.foodres.2020.109515.
We accompany you in making your products marketable. You can find out more about this under Food Safety Services.
Information on analytical services of food safety can be found here.
Last updated: 02.04.2024
automatically translated