Info for companies about cosmetics
Cosmetic products are defined as:
"substances or mixtures intended to come into external contact with the various parts of the human body (skin, hair system, nails, lips, and external intimate regions) or with the teeth and mucous membranes of the oral cavity, for the sole or principal purpose of: cleansing them, perfuming them, changing their appearance, protecting them, keeping them in good condition, or affecting body odor."
Here are links to relevant legislation and cosmetic product guidelines and recommendations. What should be considered when selling cosmetic products? What are the responsibilities of the responsible person and the trade? Important information on the product information file/safety assessment (What should a PID look like and who is allowed to create one?) and contact details.
Cosmetics law
Cosmetic products are regulated in the following laws:
- Regulation Regulation (EC) No 1223/2009 on cosmetic products applies to all cosmetic products made available in the EU and consists of 40 articles and 71 recitals, the 10 annexes are organised as follows:
| Annex I: | Safety report (safety information + assessment) |
| Annex II: | Prohibited substances |
| Annex III: | restricted authorised substances |
| Annex IV: | Colourants |
| Annex V: | Preservatives |
| Annex VI: | UV filters |
| Appendix VII: | Symbols |
| Annex VIII: | Alternative methods to animal testing |
| Annex IX: | Part A - Repealed Directive, Part B - List of deadlines for transposition |
| Annex X: | Correlation table |
- The Cosmetics Claims Regulation (EU) No. 655/2013 establishes common criteria for substantiating claims made in connection with cosmetic products. Guideline on Regulation (EU) No. 655/2013
- The Food and Consumer Protection Act (LMSVG) Federal Law Gazette I No. 13/2006 as amended regulates the requirements for cosmetic products and the associated responsibilities of business operators.
- The Cosmetics Implementing Regulation BGBl. II No. 330/2013 regulates the national regulations for the labelling of packaged and unpackaged cosmetic products.
Further guidelines and directives are:
The Austrian Food Codex (abbreviated to Codex) summarises, among other things, what is generally accepted in Austria. It is an objectivised expert opinion. It is used to publish technical terms, definitions, test methods and assessment principles as well as guidelines "for the manufacture and placing on the market of goods". Chapters relevant for cosmetics manufacturers are
- Cosmetic products B33: Natural cosmetics, rubbing alcohol, sunscreens
- Good manufacturing practice
Organic cosmetics regulation: Directive - Agricultural products from organic production and derived products (RL Biologische Produktion)
Placing on the market of cosmetics
The European Cosmetics Regulation (EC) No. 1223/2009 stipulates:
"Cosmetic products made available on the market shall be safe for human health under normal or reasonably foreseeable conditions of use, taking into account in particular: Presentation, labeling, instructions for use and disposal, any other indication or information provided by the responsible person. The affixing of warnings does not relieve the responsible person of the obligation to comply with the other requirements of the regulation."
There is no marketing authorization requirement for cosmetic products (first made available on the Community market), i.e., no pre-market regulatory review. However, all requirements and conditions of the cosmetics law (safety report, prohibited substances, etc.) must be fulfilled before placing on the market. The entrepreneur must notify his products on the European database CPNP. Importers who purchase products from third countries are legally equal to manufacturers. The imported products must therefore be manufactured and labeled in accordance with EU law (for details, see Responsible Person and Distributor Responsibilities ).
Responsible person and his tasks
The responsible person is (a legal entity or a natural person):
- the manufacturer (natural/legal person who manufactures a cosmetic product or natural/legal person who has a cosmetic product manufactured or developed and places it on the market under his own name or trademark) or
- the importer, or
- under certain circumstances, the distributor, or
- a person established within the EU to whom the manufacturer, importer or distributor passes on the responsibility with a written mandate.
According to Art. 4 and 5, the responsible person has to ensure compliance with the Regulation for each cosmetic product placed on the market under his responsibility. Obligations of the responsible person
- Safety (Art. 3) - Cosmetic products must be safe for human health under normal or reasonably foreseeable use.
- Notification (Art. 13) - Before being placed on the market, the cosmetic product must be notified to the Commission by electronic means
- Manufacture in accordance with Good Manufacturing Practice (Art. 8)
- Preparation and maintenance of the product information file and the safety assessment (Art. 10 + 11)
- CMR substances (Art. 15) and prohibited substances (Art. 14 in conjunction with Annex II) may not be used, the substances listed in Annexes III-VI only in accordance with the specified restrictions
- Nanomaterials (Art.16) - In addition to the notification according to Art. 13, cosmetic products containing nanomaterials must be notified electronically to the Commission six months before they are placed on the market.
- Labeling (Art.19) and advertising claims (Art.20).
- notification of serious undesirable effects (Art. 23)
- Information to the public on qualitative and quantitative composition and on (serious) undesirable effects (Art. 21).
In the following sections, the tasks of the responsible person are described in more detail.
Tasks of the traders
According to Article 2(1)(e), a "distributor" is any natural or legal person in the supply chain, other than the manufacturer or the importer, who makes a cosmetic product available on the Community market.
The distributor is then the responsible person (as defined in Article 4(6)) if he/she: places a cosmetic product on the market under his/her own name and trademark or modifies a product already on the market in such a way that compliance with the applicable requirements may be affected.
The translation of the labeling and information on the cosmetic product already placed on the market is not considered a change in this respect. However, the translation results in the need for notification by the distributor. Before making a product available on the market, the distributor shall verify the labeling, in particular with regard to:
- name and address, country of origin, batch number, inventory list
- whether the labeling is in German
- whether the best-before date has not expired
The trader ensures that the storage or transport conditions meet the requirements of the Ordinance. If a distributor has reason to believe that a cosmetic product does not comply with the requirements of this Ordinance,
- he/she shall not make the product available on the market
- he/she shall initiate appropriate corrective action
- immediately informs the responsible person and the competent authorities if the product poses a risk.
Cosmetic safety assessment
All cosmetic products on the market must be subjected to a safety assessment by qualified experts prior to market launch (preferably already during the development phase). Among other things, this involves a toxicological assessment of the ingredients, taking into account the exposure conditions (application concentration, application duration, application site).
Product information file
The product information file contains the following data in an easily understandable language for the competent authority of the respective member state (usually in German):
- the description of the cosmetic product
- the safety report
- the manufacturing method under the aspect of good manufacturing practice
- evidence of the claimed effect
- data on animal tests carried out
The responsible person shall make the product information file readily available to the competent authority at its address indicated on the label, in electronic or other format. The product information file shall be established and kept up to date for each individual cosmetic product made available on the market. It shall be kept for a period of ten years after the date on which the last batch of the cosmetic product was placed on the market.
Security report
The safety report (Art 10 + Annex I) is the centrepiece of the product information file. It must be prepared by the safety assessor in collaboration with the responsible person before the cosmetic product is placed on the market. The safety report consists of two parts:
1) The safety information records all essential characteristics of the cosmetic product and its ingredients that may be relevant to the safety of the product:
- Composition of the product
- Physical/chemical properties and stability
- Microbiological quality
- Impurities, traces and packaging material
- normal and reasonably foreseeable use
- Exposure to the product and the substances used
- Toxicological profile of the substances
- (serious) adverse effects
2) In the safety assessment, the safety of the cosmetic product is justified on the basis of the data in the safety report - if necessary with the addition of certain warnings.
Safety assessor and safety assessor
Safety assessments may only be prepared by persons who fulfil the following requirements (in accordance with Cosmetics Regulation (EC) 1223/2009 Article 10, paragraph 2):
"The safety assessment of the cosmetic product shall be carried out, as specified in Annex I, Part B, by a person who holds a diploma or other evidence of formal qualification awarded on completion of a theoretical and practical higher education course in pharmacy, toxicology, medicine, or a similar subject, or a course recognised as equivalent by a Member State."
A list of food and cosmetics assessors working in Austria and authorised by the Ministry of Health in accordance with § 73 LMSVG can be found in the list of the BMASGPK (cosmetics assessors are authorised for group C Z 9 "Cosmetic products" and cosmetics safety assessors also require group F Z 13 "Toxicological evaluation of goods subject to the Food Safety and Consumer Protection Act - LMSVG" (limited to goods in group C Z 9 - cosmetic products)). Prerequisites and requirements for safety assessors and experts are explained on the website of the BMASGPK.
Microbiological requirements
The assurance of microbiological quality is the responsibility of the responsible person, advised by their safety assessor. State of the art for microbiological quality for finished cosmetic products is defined in both ÖNORM EN ISO 17516: 2015 01 01 Cosmetic products - Microbiology - Microbiological limits (ISO 17516:2014) and the 11th revision of the SCCS Notes of Guidance (SCCS/1628/21, Appendix 9).
Quantitative specification
Category 1: cosmetic products intended for use in the eye area, on mucous membranes and on children under three years of age - total live germ count for aerobic mesophilic microorganisms: not more than 10² colony forming units per gram or milliliter (CFU/g or ml). Category 2: Other products - total viable count for aerobic mesophilic microorganisms: not more than 10³ CFU/g or ml.
Qualitative specification
The health safety of cosmetic products may be compromised by a variety of pathogenic agents. The indicator germs described below serve as an orientation for assessment. If there is sufficient suspicion, other pathogens should also be tested on a case-by-case basis. The following specific microorganisms must not be detectable: Pseudomonas aeruginosa, Staphylococcus aureus, Candida albicans, Escherichia coli.
Preservation
The Cosmetics Regulation (EC) 1223/2009 stipulates that in the Safety Report Part A (according to Annex I, Z3), results from preservation challenge testing are also used to assess microbiological quality. See also"Challenge testing" in the SCCS Notes of Guidance, 11th revision, Appendix 9. For further information on preservation, see"Shelf life of cosmetics".
Recommendations, publications and guides
On the subject of microbiology and hygiene of cosmetics, experts from the Microbiology and Industrial Hygiene Section of the German Society for Scientific and Applied Cosmetics (DGK e.V.) have provided a number of publications and books, which can be found here.
The Industrieverband für Körperpflege- und Waschmittel e. V. (IKW) has published a very detailed guide for Microbiological Quality Management (MQM) of cosmetic products (IKW 1998).
The following Ö-NORMEN can be obtained here:
- Cosmetic products - Microbiology - Guidance for risk assessment and identification of low-risk microbiological products (ISO 29621:2010) ÖNORM EN ISO 29621: 2011 03 01
- Cosmetics - Microbiology - Microbiological limits (ISO 17516:2014) ÖNORM EN ISO 17516: 2015 01 01
- Cosmetics - Microbiology - General guidance for microbiological testing (ISO 21148:2005) ÖNORM EN ISO 21148: 2009 10 01
- Cosmetics - Microbiology - Enumeration and detection of aerobic mesophilic bacteria (ISO 21149:2006) ÖNORM EN ISO 21149: 2009 10 01
- Cosmetic products - Microbiology - Detection of Staphylococcus aureus (ISO/FDIS 22718:2015) ÖNORM EN ISO 22718: 2015 08 01
- Cosmetic products - Microbiology - Detection of Pseudomonas aeruginosa (ISO/FDIS 22717:2015) ÖNORM EN ISO 22717: 2015 08 01
- Cosmetic products - Microbiology - Detection of Candida albicans (ISO/FDIS 18416:2015) ÖNORM EN ISO 18416: 2015 08 01
- Cosmetic products - Microbiology - Detection of Escherichia coli (ISO/FDIS 21150:2015) ÖNORM EN ISO 21150: 2015 08 01
- Cosmetics - Microbiology - Detection of specified and unspecified microorganisms (ISO 18415:2007, corrected version 2007-12) ÖNORM EN ISO 18415: 2011 07 15
- Cosmetics - Microbiology - Counting of yeasts and molds (ISO 16212:2008) ÖNORM EN ISO 16212: 2011 07 15
- Cosmetic products - Microbiology - Evaluation of antimicrobial protection of a cosmetic product (ISO 11930:2012, corrected version 2013-05-01) ÖNORM EN ISO 11930: 2014 03 01
Notify
Every cosmetic product must be notified electronically by the responsible person to the European Commission before being placed on the market (Art. 13).
The"Cosmetic Products Notification Portal (CPNP)" was installed to carry out the notification.
The user manual CPNP (Cosmetic Products Notification Portal) for responsible persons and distributors (German version, last update: 07.03.2018) helps with the registration and entry of your cosmetic products. Further German-language instructions on registration and use of the notification portal are available for download on the website of the Federal Ministry of Labour, Social Affairs, Health, Care and Consumer Protection (BMASGPK).
If a distributor translates an element of the labelling of a cosmetic product that has already been placed on the market in another Member State on their own initiative, they must also notify certain data to the European Commission electronically (see Art. 13 Para. 3 of Regulation (EC) No. 1223/2009).
Good manufacturing practice of cosmetics
Cosmetic GMP (Good Manufacturing Practice) refers to behavioral measures and regulations that must be observed and complied with in the manufacture of cosmetic products, with the aim of reproducibly producing these products in the desired quality.
The legal obligation to apply the principles of "Good Manufacturing Practice" results from Article 8 of the Cosmetics Regulation (EC) No. 1223/2009.
How to implement a Good Manufacturing Practice can be found in the following documents and guidelines:
- Cosmetics - Good Manufacturing Practices (GMP) - Guidelines on Good Manufacturing Practices (EN ISO 22716:2007) or Cosmetics - GMP - Guidelines on Good Manufacturing Practices (ÖNORM EN ISO 22716).
- Guideline of the Austrian Codex Commission for the manufacture of cosmetic products according to the principles of "Good Manufacturing Practices" (Cosmetic GMP). In the preparation of this guideline, some definitions of terms and some content points of "Cosmetics-GMP-Guideline on Good Manufacturing Practices" ISO/TC 217 N78, which is recognized at the international level, were used.
The following book on microbiology and industrial hygiene can be used as support:
- DGK Handbook"Industrial Hygiene in Cosmetics" - 2nd revised edition 2019. publishing house for chemical industry, H. Ziolkowsky GmbH, D-86470 Thannhausen/Burg, ISBN 978-3-87846-07-8.
The requirements for a microbiologically and hygienically flawless product are becoming ever more stringent. This book was written from the microbiological or hygienic point of view and is intended to provide practitioners in factories, such as plant managers, developers and those responsible for quality assurance and testing, as well as specialists in other fields, with procedural instructions and practical advice on the implementation of industrial hygiene. All the important aspects of industrial hygiene are brought together here to provide a good overview of the current state of knowledge in the field.
The name or company name and address of the responsible person. The information may be abbreviated, provided that this person and his address can be identified from the abbreviation. At this stage, an Internet address alone is not sufficient. The postal address with street, postal code and city name must be given. If more than one address is given, the address of the responsible person where the product information file is made readily available shall be highlighted (e.g. underlined). The country of origin must be indicated if the product is placed on the Community market from a third country. The form in which this information must be provided is not regulated, e.g. "Made in China".
The nominal content at the time of filling, as an indication of weight or volume, with the exception of packages containing less than 5 g or less than 5 ml, free samples and single-use packages; in the case of prepackages which are usually sold as large packages containing several pieces and for which the indication of weight and volume is not important, the indication of the content is not required, provided that the number of pieces is indicated on the package. The indication of the number of pieces is not required if it is easily recognizable from the outside or if the product is usually sold only as a unit.
The date until which the cosmetic product, when properly stored, fulfills its original function and, in particular, is compatible with Article 3 ("Best before date"). The date itself or the reference to the place where it appears on the packaging shall be preceded by the symbol of the hourglass or the words: 'Best before', as indicated in point 3 of Annex VII to Regulation (EC) 1223/2009 .
The date of minimum durability shall be clearly indicated and shall be composed of either the month and the year or the day, the month and the year, in that order. This information shall be supplemented, where necessary, by an indication of the storage conditions which must be fulfilled in order to ensure the indicated shelf life. For cosmetic products with a minimum durability of more than 30 months, the indication of the date of minimum durability is not required. For such products, it is indicated how long the product is safe after opening and can be used without harm to the consumer. For such products, except where the concept of shelf life after opening is not relevant, the length of time the product is safe after opening shall be indicated. This information shall be indicated by the symbol of the opened jar of cream shown in point 2 of Annex VII , followed by the period (expressed in months and/or years).
The ingredients shall be indicated using the common ingredient name according to the glossary published in the Official Journal of the EU: Commission Implementing Decision (EU) 2022/677 of 31 March 2022. If no common ingredient name is available, a name from a generally accepted nomenclature shall be used.
Supportive and clearer is the Commission's database for cosmetic ingredients: CosIng
The ingredients are listed using the International Nomenclature for Cosmetic Ingredients (INCI). However, CosIng does not represent a positive list of cosmetic ingredients, but is merely a collection of ingredients named by the industry.
In addition, 26 perfume ingredients (allergenic fragrances) must be declared due to their allergenic potential above a certain concentration. More on this topic, see Allergenic fragrances
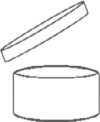
The symbol of the opened cream jar indicates the period of time during which the cosmetic product can still be considered safe after opening the product (shelf life after opening or period after opening - PAO). This period is indicated in months or years in or next to the jar symbol. Months are abbreviated with "M" and years with "J". It must be declared both on the container and on the outer packaging.

Sunscreen products with this symbol (circle with inscription UVA) declare that the cosmetic product in question complies with the EU recommendations on the minimum efficacy of photoprotection against UV-B and UV-A radiation. See "Commission Recommendation 2006/647/EC on the efficacy of sunscreen products and related claims made by the manufacturer.
Allergenic fragrances
According to Cosmetics Regulation (EC) No. 1223/2009 (Article 19), 26 ingredients (Annex III) must also be declared due to their allergenic potential.
It is not sufficient to declare these under the collective term "perfume" or herbal preparations such as essential oils and extracts if the individual substances are contained in a concentration of more than 0.001 % for products that remain on the skin/hair ("leave-on" products) or 0.01 % for products that are rinsed off ("rinse-off" products).
| No. No. | INCI name | other names | CAS no. |
|---|---|---|---|
| 45 | Benzyl Alcohol | 100-51-6 | |
| 67 | Amyl cinnamal | 122-40-7 | |
| 69 | Cinnamyl alcohol | 104-54-1 | |
| 70 | Citral | 5392-40-5 | |
| 71 | Eugenol | 97-53-0 | |
| 72 | Hydroxycitronellal | 107-75-5 | |
| 73 | Isoeugenol | 97-54-1 | |
| 74 | Amylcinnamyl alcohol | 101-85-9 | |
| 75 | Benzyl salicylate | 118-58-1 | |
| 76 | Cinnamal | 104-55-2 | |
| 77 | coumarin | 91-64-5 | |
| 78 | geraniol | 106-24-1 | |
| 80 | Anise Alcohol | Anisyl alcohol | 105-13-5 |
| 81 | Benzyl cinnamate | 103-41-3 | |
| 82 | Farnesol | 4602-84-0 | |
| 84 | Linalool | 78-70-6 | |
| 85 | Benzyl benzoate | 120-51-4 | |
| 86 | Citronellol | 106-22-9 | |
| 87 | Hexyl cinnamal | Hexyl cinnamaldehyde | 101-86-0 |
| 88 | limonene | d-Limonene | 5989-27-5 |
| 89 | Methyl 2-octynoate | Methyl heptine carbonate | 111-12-6 |
| 90 | Alpha-isomethyl ionone | 127-51-5 | |
| 91 | Evernia Prunastri Extract | Oak moss extract | 90028-68-5 |
| 92 | Evernia Furfuracea Extract | Tree moss extract | 90028-67-4 |
The following two allergenic fragrances have been included in the Prohibited List, Annex II, and may no longer be used in cosmetic products:
*) The fragrance "hydroxyisohexyl 3-cyclohexene carboxaldehyde" (Annex II/ No. 1380) has been banned due to its sensitising properties. This substance has been banned since 23 August 2021 (Regulation (EU) No. 2017/1410)
**) Butylphenyl methylpropional (Annex II/ No. 1666) was banned on 1 March 2022 due to its classification as CMR (reprotoxic) (Regulation (EU) No. 2021/1902)
The new regulations on fragrance labelling can be found here.
Manufacturers are granted a transitional period of three or five years to change the information on cosmetic products:
- For all new products (placing on the market): 3 years (31 July 2026)
- For all products already on the market (making available on the market): 5 years (31 July 2028)
Advertising claims of cosmetics
Advertising claims must be useful, understandable and reliable and enable end consumers to make an informed purchasing decision.
The requirements for advertising are regulated in Regulation (EC) No 1223/2009 Article 20 "Advertising claims" and Regulation (EU) No 655/2013 establishing common criteria for substantiating advertising claims in relation to cosmetic products.
In the labeling, making available on the market and advertising of cosmetic products, these two regulations prohibit the use of texts, designations, trademarks/brands, illustrations and other pictorial or non-pictorial signs that suggest characteristics or functions that the products in question do not possess.
List of criteria for advertising claims
Regulation (EU) No. 655/2013 includes the following criteria on advertising claims according to the Annex:
- Representations about the effects of a product must not go beyond what the available evidence supports.
- Advertising statements must not attribute any special or unique properties to the product if similar products have the same properties (advertising with self-evidence).
- If the effect of a product is linked to certain conditions, this must be clearly stated, e.g. use together with other products.
- No advertising that product has been approved or authorized by a public authority.
- Admissibility of the advertising statement is based on how the average end consumer (reasonably well informed, attentive and critical) perceives this statement, taking into account social, cultural and linguistic factors
- No advertising that ascribes a specific benefit to a product, but that benefit consists only of compliance with the minimum legal requirements
- No advertising that product has been approved or authorized by a public authority.
- Admissibility of the advertising statement is based on how the average end consumer (reasonably well informed, attentive and critical) perceives this statement, taking into account social, cultural and linguistic factors
- No advertising that ascribes a specific benefit to a product, but that benefit consists only of compliance with the minimum legal requirements
- Advertising claims must be supported by sufficient and verifiable evidence
- Evidence must take into account the state of the art
- Studies must be relevant to the product and claimed benefit, based on properly developed and applied methods (valid, reliable, and reproducible), and take into account ethical considerations.
- Strength of evidence must be consistent with the nature of the claims made, especially for claims where lack of efficacy could cause a safety problem.
- Clearly exaggerated claims do not need to be substantiated.
- Properties of an ingredient that relate to the final product must be substantiated by sufficient and verifiable evidence: by effective concentration of the ingredient in the product.
- Acceptability of an advertising claim is based on the weight of evidence of all available studies, data and information and is determined by the nature of the advertising claim and the general level of knowledge of the end user.
The associated guidance document ("Technical document on cosmetic claims") to Regulation (EU) No. 655/2013 includes:
- ANNEX I: Common criteria for cosmetic claims with examples and comments.
- ANNEX II: 'Best practice' for evidence to validate advertising claims.
- ANNEX III: Free from claims
- ANNEX IV: Hypoallergenic claim
Disease-related statements
If a cosmetic product advertises claims that the product is intended to cure or alleviate or prevent human disease or pathological conditions, it may fall within the definition of a drug and is consequently inadmissible (see Delineation of Cosmetic Products).
Delimitation & Borderline Manual
In some cases, the classification as a cosmetic product cannot be made without further ado. It must therefore be decided on a case-by-case basis by experts on the basis of function, composition, effect, product presentation, place of use or intended use.
Overlaps of cosmetics with other product groups such as pharmaceuticals, biocides, consumer goods, toys, foodstuffs or medical devices are possible. Cosmetics must comply with the legal definition of cosmetic products. There are also substances or mixtures that are not considered cosmetics, even though they serve to beautify. Thus, agents that are ingested, inhaled, injected or implanted into the human body are not cosmetic agents, as well as agents that have a predominantly different purpose, e.g. agents that serve predominantly as insect repellents. Also means for the care of animals are not cosmetic products according to the Cosmetics Regulation (EC) No. 1223/2009.
For more information on the delimitation of cosmetics from medicinal products and medical devices:
Persons wishing to place a product on the market may submit an application to the Federal Office for Safety in Health Care (BASG) pursuant to Section 1 (3b) of the German Medicines Act (AMG) for a determination as to whether that product falls within the definition of a medicinal product. The BASG also performs delineations of medical devices based solely on § 5a of the Austrian Medical Devices Act (BGBl. 657/1996 as amended, MPG). These orders are subject to a fee. The respective forms and details can be found here.
Criteria for demarcation
Important criteria for the delimitation can be found primarily in the decisions of the European Court of Justice (ECJ).
The European Commission has published a guideline on borderline products for cosmetic products, which was developed together with the Member States and deals with different products. This is updated regularly: Borderline products manual on the scope of application of the Cosmetics Regulation (EC) No 1223/2009 (Art. 2(1)(a)) (November 2024, Version 5.4)
There are two documents on the distinction between hand cleaners and hand sanitisers
- Guidance on the applicable legislation for leave-on hand cleaners and hand disinfectants
- Borderline working document on leave-on hand gels November 2020 (available in 23 EU languages)
Further Guidance Documents on "Borderlines" can be found here.
Natural Cosmetics & Organic Cosmetics
Environmentally friendly sustainable production, preservation of biodiversity, protection of natural resources, application of high animal welfare standards, production using natural ingredients and simple manufacturing processes are increasingly demanded by consumers also for cosmetic products. In order to protect the interests and trust of consumers and to ensure fair competition through transparency, control and traceability, it was therefore appropriate to formulate principles and rules for the production and labeling of "organic as well as natural cosmetics".
At the European level, there are no uniform, legal definitions of the terms natural cosmetics and organic cosmetics. There are various natural cosmetic and organic quality labels based on private law guidelines. The underlying criteria vary, so that these products do not meet a uniform standard. This situation is confusing for both consumers and manufacturers.
Natural cosmetics
In Austria, the Austrian Food Codex ÖLMB (Codex Alimentarius Austriacus, in short: Codex) summarizes what the consumer expects from natural cosmetics or what concerns the general perception of the market.
Since 2008, this Codex chapter on natural cosmetics has been available and, if necessary, is revised by the Cosmetics Sub-Commission to meet the expectations of consumers. On Feb. 6, 2017, dehydroacetic acid was included as a permitted preservative. For the full text, see Codex Chapter B 33 Cosmetic Products, Subchapter: Natural Cosmetics.
Organic cosmetics
Organic cosmetics, regulated in the EU Quality Regulations Implementation Act (EU-QuaDG), Federal Law Gazette I No. 130/2015 and the corresponding directive, which has been valid since 1.12.2017 and replaces Codex Chapter A8 Section 6 Organic cosmetics.
With the entry into force of the EU-Quality Regulations Implementation Act (EU-QuaDG), the content of Codex Chapter A 8 "Agricultural products from organic production and derived products" has been incorporated into the Guideline prepared by the Advisory Board for Organic Production in accordance with Section 13 of the EU-Quality Regulations Implementation Act (EU-QuaDG), Federal Law Gazette I No. 130/2015. The guideline - Agricultural products from organic production and derived products (RL Biologische Produktion) has the effect of an objectified expert opinion, as was already the case with the Austrian Food Book.
Labeling and advertising of cosmetic products produced according to the specifications of this section contain a clear reference to the production according to this guideline (produced according to the guideline of the Advisory Board for Organic Production "Biological Production, Section Biocosmetics").
Cosmetic companies placing their products on the market as organic cosmetics shall have their activities controlled by an organic control body already recognized for organic production under Regulation (EC) No 834/2007. The indication of the inspection body is required and the inspection body seal may be affixed, but the organic community logo may not, as the EU Organic Regulation does not apply to cosmetic products.
The natural ingredients in organic cosmetics are divided into natural substances of agricultural origin (plant and animal products) and those of non-agricultural origin (mineral raw materials and water).
Plant and animal ingredients of agricultural origin, must comply at least 95% with the provisions on organic production (Regulation (EC) No 834/2007 including implementing regulations). In addition, there must be a minimum organic content in relation to the final product (see table below). This is to counteract unjustified organic pricing, for example in the case of an aqueous solution with a "homeopathic" amount of a raw material in organic quality.
| Category | Minimum organic ingredient in % related to the finished product |
|---|---|
| Oils/water-free cleaning and care products | 90 |
| Perfumes/Eau de Parfum/Eau de Toilette | 60 |
| Emulsions for skin care (W/Ö) | 30 |
| Products with a mineral content greater than 80 | 10 |
| Other products | 20 |
Table: Biological minimum proportions in organic cosmetics
Furthermore, rules have been laid down as to the proportion of aqueous mixtures such as distillates, extracts, hydrolates or even rediluted concentrates that may be used to calculate the organic content. Only the proportions actually obtained from plants are to be included in the organic content.
As in the case of natural cosmetics, only natural fragrances and aromatic substances that comply with the international standard ISO 9235 and the substances listed therein that have been isolated by physical methods may be used. The permitted nature-identical preservatives are also those that may be used in natural cosmetics. For a list on naturident preservatives, see Codex Chapter B 33 Cosmetic Products, Subchapter: Natural Cosmetics.
The full text of the directive can be found under: Directive - Agricultural products from organic production and derived products (RL Biologische Produktion) (Section 6 Biokosmetik).
Adverse effects
Article 2 of Regulation (EC) No 1223/2009 defines the following terms:
An undesirable effect is an adverse effect on human health attributable to the normal or reasonably foreseeable use of a cosmetic product.
A serious adverse effect is an adverse effect that results in temporary or permanent functional impairment, disability, hospitalization, congenital anomalies, imminent risk to life, or death.
According to Article 23, the responsible person, distributors and dealers must immediately report the occurrence of serious adverse effects to the competent authority. There is also an option for consumers and physicians to report serious adverse effects.
| Reporting forms | for whom? | Report to |
|---|---|---|
| Reporting form A | responsible person and the distributor | competent authority |
| Notification form AT | consumers and physicians | competent food supervisory authority |
Each form is accompanied by instructions for completion. The submitted form is to be used for both initial reporting and updates (follow-up, final conclusion).
The Guideline for Reporting Serious Adverse Effects describes in detail how to proceed and how to perform the causality assessment. Serious adverse reactions and adverse effects shall also be reported in the safety information and product information file (see Cosmetic Product Safety Assessment) (Article 11).
Furthermore, the responsible person shall ensure that existing data on undesirable effects and serious undesirable effects caused by the cosmetic product during its use are made readily available to the public by appropriate means (Article 21)
On the homepage of the Ministry of Social Affairs you can find more detailed information on the reporting system of serious undesirable effects as well as the contact points of the respective food inspectorate in your federal state.
For more information on Serious Undesirable Effects (SUE), please visit the European Commission's page on cosmetics.
Further information
- Federal Office of Consumer Protection and Food Safety
- Federal Institute for Risk Assessment BfR
- Chemical and Veterinary Investigation Offices CVUA
- German Society for Scientific and Applied Cosmetics DGK (see expert groups)
- German Chemical Society GDCh: Working Group on Cosmetic Products (see "Data Sheets for the Evaluation of the Efficacy of Active Ingredients in Cosmetic Products")
- German Industrial Association for Personal Care and Detergents IKW: Beauty Care
- Guideline on the management of adverse effects and the reporting of serious adverse effects within the European Union (Cosmetics Europe/IKW 2013).
- Colipa guidance on product information file requirements - PID (Cosmetics Europe/IKW 2013).
- Colipa Guidance: Responsibilities within the supply chain (Cosmetics Europe/IKW 2013).
- Colipa guidelines on cosmetic product labeling (Cosmetics Europe/IKW 2013).
- Database of the European Commission: CosIng (fast, clear search for cosmetic ingredients)
- European Commission: Cosmetic Products
- European Cosmetics Regulation (Regulation (EC) No. 1223/2009 - consolidated version)
- European Cosmetic Association - Cosmetics Europe
- IFRA - International Fragrance Association
- RAPEX notifications
- Scientific Committee on Consumer Safety (SCCS) - scientific cosmetics committee, mandates and opinions (SCS-Opinions 2016-2021)
Last updated: 01.04.2025
automatically translated